Statswork Systematic review vs meta-analysis
Systematic reviews and meta-analysis, both, form a part of literature review and are often confused and used interchangeably. But this is not the case as there are distinct differences despite the apparent overlap between the two.
What is a systematic review?
A systematic review involves the identification and synthesis of scientific studies relevant to a research question to provide a comprehensive summary of all scientific evidence to the particular question. Systematic reviews result in high-quality secondary data that concretely substantiates the case made. It is an exhaustive summary of all the literature that is pertinent to the research question. The synthesis of the results of the studies can be qualitative or quantitative.
The general application of systematic review is high in healthcare industries, as it where the review originated. But it is also used in clinical trials, social studies, etc.
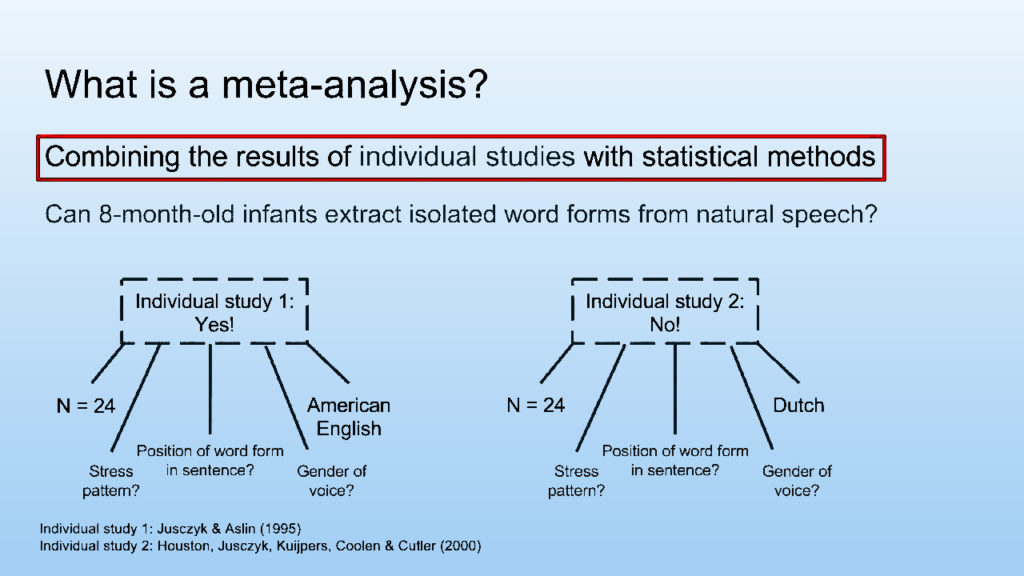
What is a meta-analysis?
Meta-analysis is the statistical integration of conceptually similar studies to attain a point estimate closest to the standard truth. It is based on the understanding that all similar studies contain a common truth between them to which all individual studies arrive with a degree of error. Thus, combining the results of various studies will provide an estimate of this common value. Meta-analysis is a subset of systematic review which identifies, selects, and combines the results of studies and applies statistical principles to achieve a pooled estimated with a higher power of statistical certainty.
Meta-analysis can identify if a study shows more variation than what is expected. Thus, weakness in the methodology of individual studies can be corrected.
Meta-analysis finds its applications in fields such as medicine, education, psychology, criminal justice, sociology, social psychology, finance and economics, political science, etc.
Pharmaceutical companies use meta-analysis to gauge their model’s generalizability, developing and validating different prediction models.
Regulatory agencies use meta-analysis in the process of drug approvals.
Systematic review vs Meta-analysis:
Both systematic review and meta-analysis are an abridgement of the body of evidence for a specific question by researchers.
Meta-analysis is always quantitative while systematic reviews include both qualitative and quantitative studies. Although meta-analysis is not always conducted in the framework of systematic reviews, it most often is used in systematic reviews that involve combining studies with numerical results.
For systematic reviews whose questions are qualitative, that is, the ones that require a combination of qualitative studies meta-analysis can’t be performed.
When doing conducting a meta-analysis for a systematic review, specific considerations have to be made:
This includes identification of the relevance of studies to the question and the compatibility to perform a meta-analysis. Studies that are different can’t be combined for meta-analysis.
In short, systematic reviews and meta-analysis are both used to reduce bias in studies and provide comprehensive evidence to a question or a truth (statistical estimate). Systematic reviews, when dealing with conceptually similar quantitative studies apply meta-analysis to integrate results for a more accurate pool estimate. But it is not always required, and sometimes meta-analysis is conducted without the context of systematic analysis, as in the case of clinical trials. Hence, it can be said that meta-analysis and systematic review overlap and when they do the former is always a subset of the latter.


 Next Post
Next Post