Methods and techniques bridging clinical investigation and statistics while writing statistical methodology
Brief:
- A qualitative data analysis services statistician help to optimize design, analysis and interpretation of results, and concluding.
- The importance of the sample size collection is to determine the optimal number of participants to include in the trial.
- Primary and secondary data in statistical analysis needs design which is essential for every clinical study.
Statistical data analysis in qualitative market research used to evaluate the most appropriate technique should use to make the analysis. An essential feature of a clinical examination is that it aims to use results based on a sample of research to see if the intervention is sufficient or if it is comparable to analyze.The sample size is the crucial thing in the analysis process; both small and large amount of data affect the result.
It is essential to give the statistician a rough proposal format.The data analysis plan for qualitative research needs a functional protocol because any changes in the contractmay affect the required size of the sample and analysis plans. To send a copy of modifications have been made to the study design
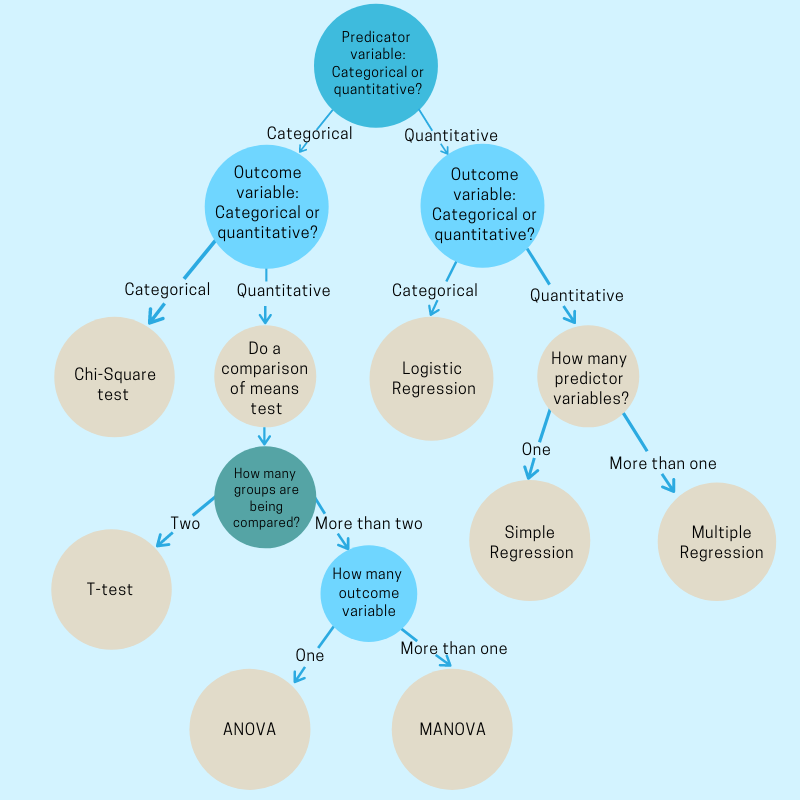
Study design:
The starting stage,the organization should give the proper configuration for the study to conduct. There are various types of study design present it varies based on the study going to lead. In most of the cases, the study design is more important than analysis. A reduced study can not be retrieved, but a lousy analysis can reanalyze. It is important because the design of the study gives information about how the data should be analyzed.
Sample size:
Most common thing is the sample size, it is essential because if the study issmall, it will not be able to answer the question proposed also waste time and money spent on analysis. Qualitative data analysis in market research data guides you to have an idea and choose the number to analyze. Sample sizedepends on critical quantities,
- A Type I error (alpha) is the risk of inferring a difference between study groups when there is no such difference.
- A Type II error (beta) is the risk of no difference between study groups when there is a difference.
- Statistical test’s ability to detect an actual difference. Power = 1 – Type II error.
The qualitative sample size computed to provide a fixed level of power to alter the hypothesis. Power is an important consideration. Low power can cause an actual difference in clinical outcomes between study to go undetected part. However, more power may yield statistically significant results that are not meaningfully different.
Analytical Tools for Qualitative Research assist in sample size planning for the clinical study based on the estimation from the prior information which has different precision.It considered while interpreting the result.A clinical trialconducted without a sample size or power information takes the risks of either failing to detect clinically meaningful differences.
Determining a reasonable and affordable size estimate is a team effort—quantitative data analysis in business research statistician help to solve practical issues such as budgets and assist in having limitations in the investigation. Too large sample size could preclude the ability to conduct the research.The research team will assess scenarios with varying detectable differences and power as sample size per group and the detectable difference.
Statistical analysis methodology:
The Statistical analysis methods for analyzing the study outcomes are to be carefully detailed. Online company and management data collection tools help in specifying these methods in advance is another way to minimize bias and maintain the integrity of the analysis. There are two main study methods descriptive statistics describe data from a sample using indexes as mean, standard deviation, which concludes data that are subject to random variations. Descriptive statistics have two sets of properties that are distribution and dispersion.Qualitative factors in business decision making provide standard statistical procedure involves the collection of leading data set to test the relationship of two statistical data.
The need for a statistician in the clinical investigation:
- To clarify the research questions. And to investigate whether the primary hypothesis is clear.
- Qualitative and mixed methods dissertation help identify the data outcome related to the research questions. To make sure the primary and secondary information clearly stated.
- They check for the study design has addressed the hypothesis.
- They analyze the assumption used in sample size estimation indicated with the reference.
- They make changes in the data analysis plan to get a good outcome
References:
- Adams-Huet, B., & Ahn, C. (2009). Bridging clinical investigators and statisticians: writing the statistical methodology for a research proposal. Journal of Investigative Medicine, 57(8), 818–824.
- Bridging Clinical Research & Clinical Health Care. (2020). Insights From the Bridging Clinical Research & Clinical Health Care Collaborative.
- Hartling, L., Scott-Findlay, S., Johnson, D., Osmond, M., Plint, A., Grimshaw, J., & Klassen, T. P. (2007). Bridging the Gap between Clinical Research and Knowledge Translation in Pediatric Emergency Medicine. Academic Emergency Medicine, 14(11), 968–977.



 Previous Post
Previous Post Next Post
Next Post